| Sign In | Join Free | My carsrow.com |
|
- Home
- Products
- About Us
- Quality Control
- Contact Us
- Get Quotations
| Sign In | Join Free | My carsrow.com |
|
Brand Name : New Life
Model Number : Cassette
Certification : ISO13485,CE
Place of Origin : China
MOQ : 5000pcs
Payment Terms : T/T, Western Union
Supply Ability : 2000000pcs/month
Delivery Time : 20-30days
Packaging Details : 1pc/pouch, 25pcs/box
Category : Disease
Format : Strip/cassette
Specimen : Swab
Testing time : 5-15 minutes
Shelf Life : 24 Months
Application : Mycoplasma pneumoniae Antigen
Accuracy : 98.15%
4mm cassette Mycoplasma pneumoniae Antigen Rapid Diagnostic test , quickly and easily, gold colloidal method
Accessories:
| Test Cassettes | Sterilized Swabs | Sample Extraction Buffer |
| Tube Tips | Workstation | Package Insert |
| Extraction Tubes |
Intended Use:
Mycoplasma pneumoniae Antigen Rapid Test Cassette is a rapid chromatographic immunoassay for the qualitative detection of Mycoplasma pneumoniae(M. pneumoniae) antigens in human throat swabs. It is intended to aid in the rapid differential diagnosis of Mycoplasma pneumoniae infections.
TEST PRINCIPLE
The Mycoplasma pneumoniae Antigen Rapid Test Cassette a qualitative, lateral flow immunoassay for the detection of M. pneumoniae antigen in a throat swab. In this test, antibody specific to M. pneumoniae antigen is coated on the test line region of the test. During testing, the extracted throat swab specimen reacts with an antibody to M. pneumoniae that is coated onto particles. The mixture migrates up the membrane to react with the antibody to M. pneumoniae on the membrane and generate a color line in the test line region. The presence of this color line in the test line region indicates a positive result, while its absence indicates a negative result. To serve as a procedural control, a colored line will always appear in the control line region, indicating that proper volume of specimen has been added and membrane wicking has occurred.
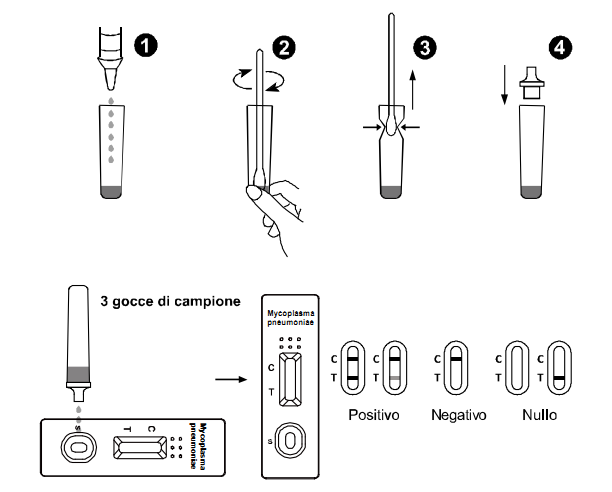
TEST PROCEDURE
Allow the test, specimen, extraction buffer to equilibrate to room temperature (15-30°C) prior to testing.
Remove the test cassette from the sealed foil pouch and use it as soon as possible. Best results will be obtained if the assay is performed immediately after opening the foil pouch.
Caution: The above interpreting time is based on room temperature range of 15-30℃. If your room temperature is significantly lower than 15℃, then the interpreting time should be properly increased to 30 minutes.
INTERPRETATION OF RESULTS
Positive:
Two red lines are visible in the result window. The intensity of the test line may be
weaker or darker than that of the control line. This still means a positive result.
Negative:
The control line appears in the result window, but the test line is not visible.
Invalid:
If the control line does not appear in the result window, the test results are INVALID regardless of the presence or absence of the line in the test region.
PERFORMANCE CHARACTERS:
Sensitivity, Specificity and Accuracy
The Mycoplasma pneumoniae Antigen Rapid Test Cassette (Throat Swab) has been evaluated with specimens obtained from the patients. PCR is used as the reference method for the Mycoplasma pneumoniae Antigen Rapid Test Cassette (Throat Swab). Specimens were considered positive if PCR indicated a positive result. Specimens were considered negative if PCR indicated a negative result.
| Method | PCR | Total Result | ||
| Mycoplasma pneumoniae Antigen Rapid Test Cassette(Throat Swab) | Results | Positive | Negative | |
| Positive | 33 | 3 | 36 | |
| Negative | 2 | 233 | 235 | |
| Total Result | 35 | 236 | 271 | |
Relative sensitivity: 94.3% (95%CI*: 80.8%~99.3%);
Relative specificity: 98.7% (95%CI*: 99.6%~100.0%);
Accuracy: 98.2% (95%CI*: 95.7%~99.4%). *Confidence Intervals
NOTE: Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for the control line failure. Review the procedure and repeat the test with a new device. If problem persists, please contact your local distributor.
| ORIENT NEW LIFE MEDICAL CO., LTD. | |
| Contact: | Jerry Meng |
| Email: | Jerry @ newlifebiotest .com |
| Tel. | +86 18657312116 |
| SKYPE | enetjerry |
|
|
Swab Specimen Infectious Disease Blood Tests , Self Blood Test Kit For M. Pneumoniae Antigens Images |